Pneumonia is the main cause of death in HIV-infected children. A significant number of undiagnosed or poorly treated HIV-infected children present to health services with severe pneumonia. WHO guidelines to treat severe pneumonia in HIV-infected infants include empirical treatment against common bacteria plus Pneumocystis jirovecii. Although this approach has contributed to reduce overall case fatality rates, autopsy studies have shown that mortality in this particularly vulnerable group remains unacceptably high. My previous autopsy studies in Africa have shown that cytomegalovirus (CMV) infection and tuberculosis are important underdiagnosed and undertreated causes of death, each accounting for up to 20% of mortalities in HIV-infected infants. Un-controlled studies from South Africa have shown that ganciclovir can successfully treat cytomegalovirus pneumonia, but there have been no clinical trials demonstrating efficacy in HIV-infected African children to date. The prevalence of unrecognized tuberculosis in HIV-infected infants with severe pneumonia has been estimated to be around 18%. However, this is likely an underestimate, as >50% of children who die of tuberculosis had not been diagnosed ante-mortem. Despite the high mortality associated with tuberculosis and cytomegalovirus, in many resource-limited settings there is no diagnosis in place and available diagnostic tools perform poorly.
Pneumonia is the main cause of death in HIV-infected children. A significant number of undiagnosed or poorly treated HIV-infected children present to health services with severe pneumonia. WHO guidelines to treat severe pneumonia in HIV-infected infants include empirical treatment against common bacteria plus Pneumocystis jirovecii. Although this approach has contributed to reduce overall case fatality rates, autopsy studies have shown that mortality in this particularly vulnerable group remains unacceptably high. My previous autopsy studies in Africa have shown that cytomegalovirus (CMV) infection and tuberculosis are important underdiagnosed and undertreated causes of death, each accounting for up to 20% of mortalities in HIV-infected infants. Un-controlled studies from South Africa have shown that ganciclovir can successfully treat cytomegalovirus pneumonia, but there have been no clinical trials demonstrating efficacy in HIV-infected African children to date. The prevalence of unrecognized tuberculosis in HIV-infected infants with severe pneumonia has been estimated to be around 18%. However, this is likely an underestimate, as >50% of children who die of tuberculosis had not been diagnosed ante-mortem. Despite the high mortality associated with tuberculosis and cytomegalovirus, in many resource-limited settings there is no diagnosis in place and available diagnostic tools perform poorly.
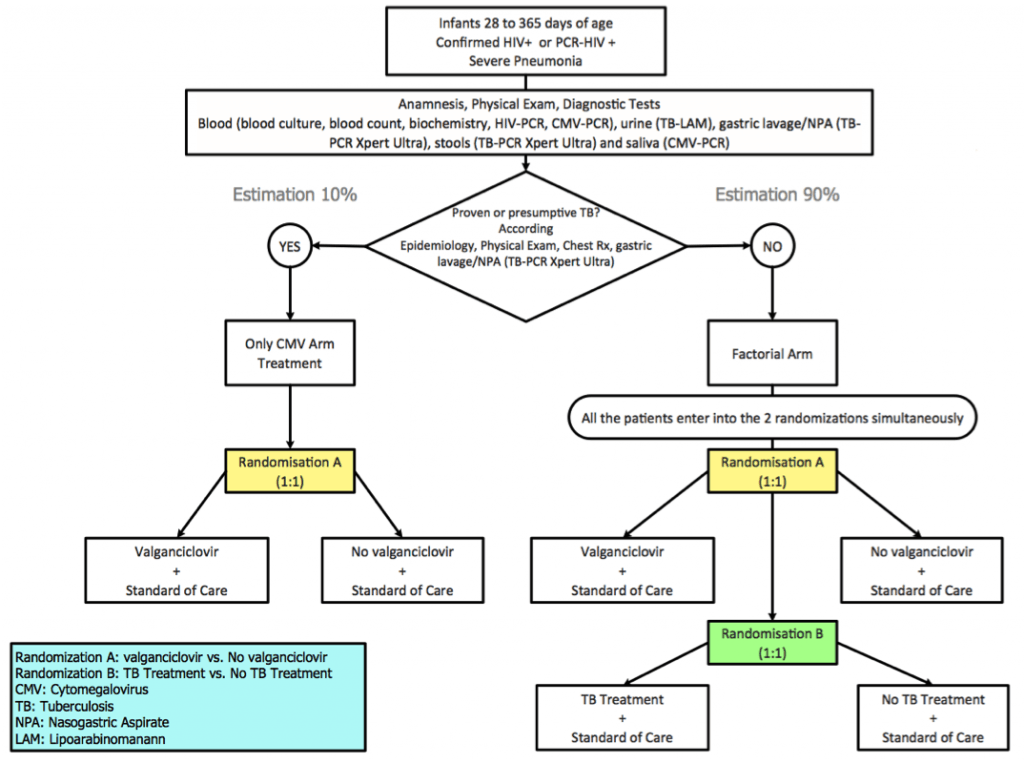
The objective of the ‘EMPIRICAL’ clinical trial is to evaluate whether empirical treatment against cytomegalovirus and tuberculosis improves survival of HIV-infected infants with severe pneumonia. A randomized factorial clinical trial will be conducted in six sub-Saharan African countries to evaluate the safety and efficacy of empirical treatment against cytomegalovirus and tuberculosis in HIV-infected infants aged 1 month-12 months admitted to hospital with severe pneumonia. Study end-points include 15-day and 12-month survival. HIV-infected infants will receive standard of care (SoC) pneumonia treatment including antibiotics, cotrimoxazole and prednisolone. Infants with presumptive tuberculosis will receive tuberculosis treatment and will be randomized to receive either SoC plus valganciclovir or SoC. Patients with a diagnosis of non-presumptive tuberculosis will be randomized to receive SoC plus valganciclovir and tuberculosis treatment in a 2×2 factorial randomization. Causes of death among study participants will be studied using verbal autopsies and minimally invasive autopsies. A drug-to-drug interaction sub-study of antiretrovirals/rifampicin will be assessed in a sub-sample of participants. This trial is funded by the EDCTP (European & Developing Countries Clinical Trials Partnership) and incorporates aspects that address the management and optimization of three priority diseases: HIV, tuberculosis and lower respiratory infections, which often overlap. the EMPIRICAL trial represents an innovative management approach that is readily scalable, and could result in a decrease of mortality in this highly vulnerable population group.
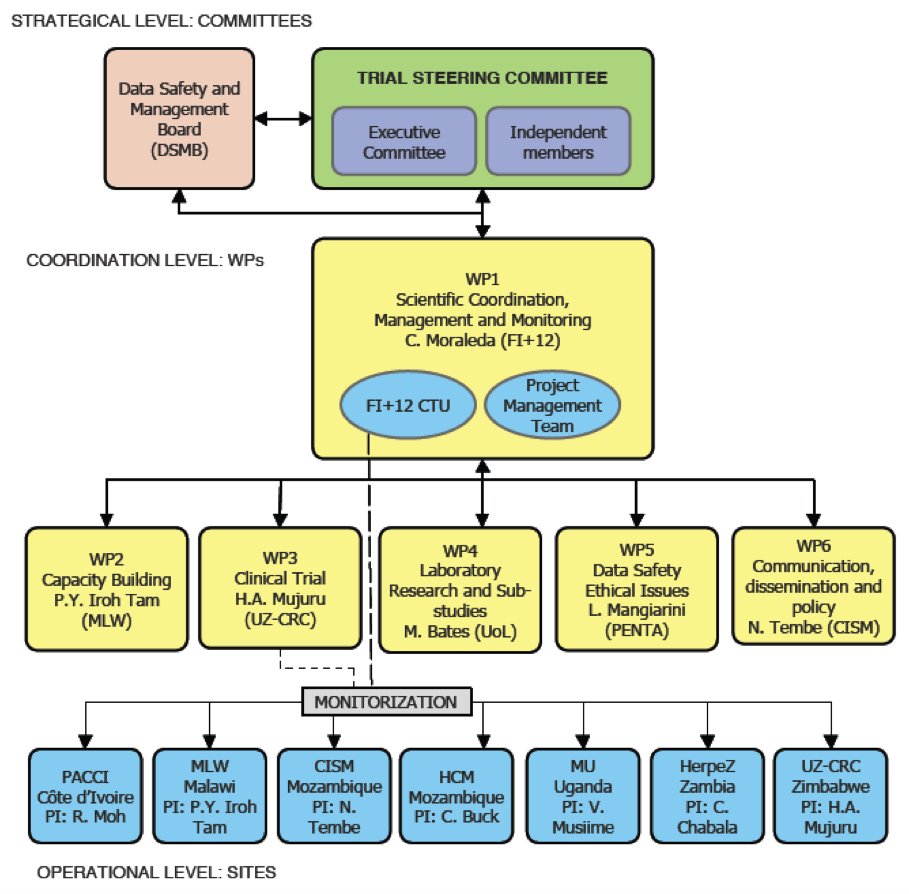
Over 600 HIV-infected infants will be recruited across 6 African countries and the University of Lincoln will lead Work Package 4, which is responsible for coordinating the laboratory diagnostic components of the trial. This represents the first high profile international clinical trial in which the University has been involved, and augers well for the opening of the University of Lincoln Medical School in Sept 2019. We hope that from this foundation we can build clinical research capacity, both through international collaborative projects, and also locally to address health challenges of importance to the local community in Lincolnshire.